Your KEYTRUDA® (pembrolizumab) Treatment
The information provided on this site is general education information and does not take the place of your healthcare professional’s advice. Please always follow your healthcare professional’s instructions and talk with him/her about any questions or problems you have regarding your health and treatment.
All the information on this page is for adults, not children and adolescents. For information for children and adolescents, please consult with your healthcare professional for more information.
What Is KEYTRUDA And What Is It Used For?
KEYTRUDA contains the active substance pembrolizumab, which is a monoclonal antibody.1 KEYTRUDA works by helping your immune system fight your cancer.
KEYTRUDA Is Used In Adults To Treat:1
- A kind of skin cancer called melanoma
- A kind of lung cancer called non-small cell lung cancer
- A kind of blood cancer called classical Hodgkin lymphoma
- A kind of cancer called bladder cancer (urothelial carcinoma)
- A kind of head and neck cancer called head and neck squamous cell carcinoma
- A kind of kidney cancer called renal cell carcinoma
- A kind of cancer that is determined to be microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) in the colon or rectum (called colorectal cancer), uterus (called endometrial cancer), stomach (called gastric cancer), small bowel (called small intestine cancer), bile duct or gallbladder (called biliary tract cancer)*
- A kind of cancer called oesophageal carcinoma
- A kind of breast cancer called triple-negative breast cancer
- A kind of uterine cancer called endometrial carcinoma
- A kind of cancer called cervical cancer
*MSI-H/dMMR can occur when a cell is unable to repair mistakes made during the division process, resulting in errors in the DNA to start accumulating, which may cause cancer.2
People receive KEYTRUDA when their cancer has spread or cannot be taken out by surgery.1
People receive KEYTRUDA after they have had surgery to remove melanoma, non-small cell lung carcinoma or renal cell carcinoma, to help prevent their cancer from coming back (adjuvant therapy).1
People receive KEYTRUDA before surgery (neoadjuvant therapy) to treat triple-negative breast cancer, and then continue taking it after surgery (adjuvant therapy), to help prevent their cancer from coming back.1
If you notice any symptoms while receiving your pembrolizumab treatment, you should speak to your healthcare professional right away. Be aware that side effects may still occur after receiving the final dose of your treatment and can affect more than one body area. Certain medicines, such as corticosteroids, may be used to help prevent more severe complications and reduce your symptoms. Your healthcare professional may delay or completely stop your treatment if your side effects are too severe. Do not attempt to diagnose or treat side effects yourself.1
Ensure that you read the Patient Safety Information Brochure and carry your Patient Alert Card with you at all times.
How Does My Treatment Work?
Normally, our bodies are good at finding cancer and destroying it. However, some cancers can send out signals that help them hide from our immune systems. This lets cancer cells grow and spread.3
How KEYTRUDA Works3
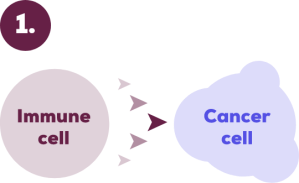
The immune system is your body’s natural defence against disease.
It sends immune cells throughout your body to seek out cancer cells and destroy them.
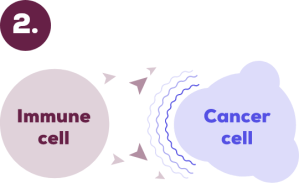
However, some cancer cells can make signals which let them hide from our immune system.

KEYTRUDA helps to stop these signals.
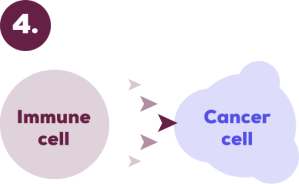
Your immune system can once again identify and attack the cancer cells.
This helps your immune system do what it’s meant to: detect and attack the cancer cells.
KEYTRUDA is sometimes given in combination with chemotherapy and/or other anti-cancer treatments: tyrosine kinase inhibitors (TKIs) and bevacizumab depending on your tumour type.
How The Combination Of KEYTRUDA And Chemotherapy Works
Chemotherapy is a type of anti-cancer treatment that uses powerful chemicals to kill cancer cells, which grow and multiply much quicker than most cells in the body.
How Chemotherapy Works
Chemotherapy drug
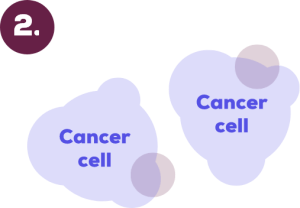
Cancer cells attacked by chemotherapy drug
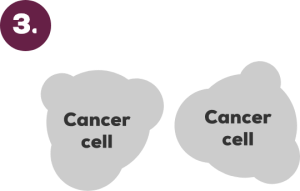
Chemotherapy drug can kill cancer cells by damaging their DNA or by stopping them from dividing and growing
When KEYTRUDA is used in combination with chemotherapy, your treatment can enhance your body’s immune response and increase cancer cell death.
In some cases, you may be prescribed KEYTRUDA with chemotherapy and bevacizumab depending on your tumour type. In addition to the above, bevacizumab may help by reducing blood supply to cancer cells in a similar way to how TKIs work, as described below.4
How The Combination Of KEYTRUDA And A TKI Works3
KEYTRUDA and TKIs affect two different ways that cancer grows. TKIs are a type of drug that works by reducing the blood supply to cancer cells. This means cancer cells don’t have enough food and oxygen, which causes them to die.
How TKIs Work
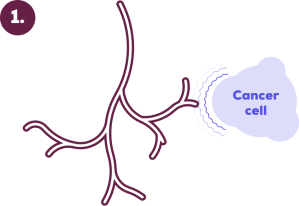
Cancers can send out signals that cause new blood vessels to grow towards the cancer.
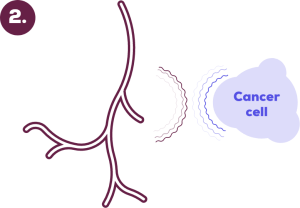
TKIs can block these signals. This stops blood vessels from growing towards the cancer and starves cancer cells of food and oxygen.
When KEYTRUDA is used in combination with a TKI (axitinib or lenvatinib), your treatment can enhance your body’s immune response and also help reduce the blood supply to cancer cells.
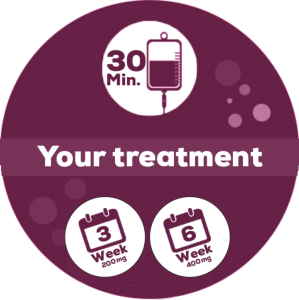
How Is My Treatment Given?
Monotherapy3
KEYTRUDA will be given to you in a hospital or clinic under the supervision of an experienced healthcare professional. KEYTRUDA will be given through an infusion into a vein (intravenous drip) every 3 weeks or every 6 weeks. It takes about 30 minutes to get each infusion. Nurses will help connect the drip and make sure that the solution is flowing correctly and safely.
In Combination With Chemotherapy3
You will usually receive your chemotherapy treatment at a day unit or in hospital. The number of times you will be given chemotherapy will depend on exactly what your doctor has prescribed for you. It is often given every 3 to 4 weeks for a few months. There are different ways of having chemotherapy drugs. They are often infused into a vein (intravenous drip) over several hours. Your doctor will talk to you about the most suitable option for you.
| KEYTRUDA | Chemotherapy | |
|---|---|---|
| How? | Infusion | Infusion (or sometimes, tablets) |
| When? | Once every 3 weeks or once every 6 weeks | Commonly once every 3 to 4 weeks, or once a week for 3 weeks and then a rest for a week, for a few months |
| Where? | Usually in a clinic, with the help of a nurse | Usually in a clinic, with the help of a nurse |
Chemotherapy With Bevacizumab4
In some cases, you may be prescribed KEYTRUDA with chemotherapy and bevacizumab. Your doctor or nurse will give you bevacizumab as a solution by intravenous infusion once every 2 or 3 weeks. The number of infusions that you receive will depend on how you are responding to treatment.
For further information, consult the bevacizumab Patient Information Leaflet.
In Combination With A TKI (Axitinib or Lenvatinib)
Axitinib5
Axitinib is a red tablet that you take by mouth 2 times a day, 12 hours apart. The standard axitinib tablet is a 5-mg dose and has a triangular shape.
Sometimes, doctors may change to a different dose of axitinib, which is in a different-shaped tablet. Your doctor, nurse or pharmacist will let you know the exact dose of axitinib that you will receive.
For further information, consult the axitinib Patient Information Leaflet.
Lenvatinib6,7
Lenvatinib is a capsule that you take by mouth. It has a yellow body and a yellowish-red cap. The standard lenvatinib dose in combination with KEYTRUDA is 20 mg (two 10-mg capsules) taken once daily.
Sometimes, doctors may change to a different dose of lenvatinib, which might involve taking two capsules of different doses once daily. Your doctor, nurse or pharmacist will let you know the exact dose of lenvatinib that you will receive.
For further information, consult the lenvatinib Patient Information Leaflet.
| KEYTRUDA | Axitinib | Lenvatinib | |
|---|---|---|---|
| How? | Infusion | Tablets, by mouth | Capsules, by mouth |
| When? | Once every 3 weeks or once every 6 weeks | 5 mg twice daily | 20 mg once daily |
| Where? | Usually in a clinic, with the help of a nurse | At home | At home |
Take your medicine as prescribed and inform your doctor, pharmacist or nurse immediately if you get any side effects. Always read the Patient Information Leaflet of any medicine you have been prescribed.
Will I Experience Side Effects?
Like all medicines, KEYTRUDA can cause side effects. Although not everyone gets them, it is important to look out for any signs and/or symptoms. Do not wait until your next appointment. If you notice any symptoms, you should let your healthcare team know straight away. This will help your doctor stop them from becoming more serious. Your doctor may give you other medicines to prevent more severe complications and reduce your symptoms.
Many of the possible side effects can be managed without having to permanently stop your treatment. Your doctor will decide how to best manage your side effects. Do not try to diagnose or treat side effects yourself. Always carry your Patient Alert Card with you and remember to read the patient safety information brochure and Patient Information Leaflet for all your medicines.
Tell your doctor if you have had a solid tumour transplant or if you’re being considered for a stem cell transplant.
Understanding Possible Side Effects
Like all medicines, KEYTRUDA can cause side effects. Although not everybody gets them, it is important to look out for any signs and/or symptoms. If you get any side effects, talk to your doctor straight away. This includes any possible side effects not listed in the KEYTRUDA Patient Information Leaflet (PIL) or in the diagram below.
Being Aware
Your healthcare team should have provided you with materials to help you identify any side effects you may experience on your treatment. It is important to be aware of side effects. You may experience more than one side effect at the same time. Telling your healthcare professional IMMEDIATELY once you notice any symptoms may stop them from becoming more serious.
Do NOT wait for your next appointment.
DO NOT ATTEMPT TO DIAGNOSE OR TREAT SIDE EFFECTS YOURSELF.
Possible Side Effects
Below is a diagram of the possible side effects that you should look out for. If you get any side effects, talk to your doctor immediately. This includes any side effects not included in the KEYTRUDA Patient Information Leaflet (PIL) or in the diagram below.

 Eyes
Eyes
- My eyesight has changed
- I have loss of vision
- My eyes hurt or feel uncomfortable
- I have noticed a yellowing of my eyes
 Mouth and head
Mouth and head
- I am more thirsty than usual
- I have a dry mouth
- My sense of taste has changed
- I have a sweet or metallic taste in my mouth
- I have a sweet smell to my breath
- I feel faint or dizzy
- I have headaches that will not go away or are unusual for me
 Skin and hair
Skin and hair
- I have noticed changes to my skin or hair
- I have noticed changes in the colour of my skin
- I have developed a rash or my skin is itchy or dry
- I have skin blistering, peeling or sores
- I have ulcers in my mouth or in the lining of my nose, throat, or genital area
- I am bleeding or bruising more easily than normal
- I am sweating more than normal
- My sweat has a different odour
- My hair is falling out
 Throat and chest
Throat and chest
- I have developed a new or worse cough
- My voice is getting deeper
- I feel more short of breath
- My breathing is faster and deeper
- I have chest pain
- I have noticed a rapid or irregular heartbeat
 Urine
Urine
- The amount, odour or colour of my urine has changed
- I need to urinate more often
- I have urinary incontinence or difficulty urinating
- It is painful when I urinate
- I have blood in my urine
 Stomach and bowels
Stomach and bowels
- I feel less or more hungry than usual
- I have been nauseous or vomiting
- I am constipated
- I have diarrhoea or more bowel movements than usual
- My stools are black, tarry, sticky or have blood or mucus
- My stomach area feels sore or tender
- I have pain or pressure in my lower abdomen
 Muscles, nerves, joints and limbs
Muscles, nerves, joints and limbs
- I have a stiff neck
- There is swelling or pain in my legs or arms
- I have muscle cramps, spasms, pain or weakness
- I have joint pains
- I feel numbness, burning, tingling and/or paralysis in my arms or legs
 General
General
- I feel more tired or confused
- I have memory problems
- I have seizures
- I have trouble sleeping
- I feel unusually sleepy
- I feel colder than normal
- I have chills or flu-like illness
- I have a fever or have hot flushes
- I have lost or gained weight
- I am feeling generally unwell
GB-PDO-02721 | Date of Preparation: April 2023
Additional Possible Side Effects With KEYTRUDA When Taken In Combination With Chemotherapy Or A TKI
KEYTRUDA in combination with chemotherapy1
- A reaction related to infusion of the chemotherapy
KEYTRUDA in combination with a TKI1
- Pain when you urinate
- Uncomfortable sensitivity to light or seeing spots
Please consult your healthcare team and read the appropriate Patient Information Leaflet for the treatment you are taking in combination with KEYTRUDA for further information related to potential additional side effects.
What Do I Do If Symptoms Occur
It is important to contact your healthcare professional as soon as symptoms occur. Always carry your Patient Alert Card which contains important information about reporting any signs of symptoms (including those not listed) immediately to your healthcare professional treating you while you are away from home. It also alerts other healthcare professionals that you are being treated with pembrolizumab. Certain medications, such as corticosteroids, may be used to help prevent more severe complications and reduce your symptoms. Your healthcare professional may delay or completely stop your treatment if your side effects are too severe.
Do not attempt to diagnose or treat side effects yourself.
Additional Resources
Sometimes, it can feel like there is a lot to remember about your treatment or condition. Having additional resources and information can help you to stay on top of the most useful things to know and can support you during your treatment. You may find some additional resources useful to keep track of how you are feeling, to record any questions that you may have for your healthcare professional and to learn more about your cancer. By following the link below you will find additional links and resources that may support you.
Your healthcare team should have provided you with a Patient Safety Information Brochure and Patient Alert Card to help you identify any side effects you may experience on your treatment. Ensure you read these materials and carry your Patient Alert Card with you at all times.
Patient Frequently Asked Questions
This section answers some of the questions you might have about your treatment. It is a good idea to jot down any other questions you have in your immunotherapy diary, so that you can ask your healthcare professional at your next appointment.
What Effect Will KEYTRUDA Have On My Other Medicines?
Your treatment may interact with other medicines. It is important to tell your healthcare professional about any medicines you are currently taking or are planning to take.
Can I Take Antibiotics?
It is important to ensure that any medications are compatible with your KEYTRUDA treatment. Ensure your healthcare professional is aware of any treatments you are currently taking or planning to take.
Can I Take Vitamins And Herbal Supplements?
You should tell your healthcare professional about all the medicines you take, including vitamins and herbal supplements. Your healthcare professional can help you to decide if they are suitable to take or not.
Can I Have Vaccinations?
Consult your healthcare professional before receiving any vaccinations, including flu or COVID vaccination.
Can I Go On Holiday?
Discuss your holiday plans with your healthcare professional before you book your holiday. Some extra preparation may be necessary, and you should always carry your Patient Alert Card with you.
Should I Change My Diet?
A healthy diet is important. You can discuss any changes to your diet with your healthcare professional.
Can I Drink Alcohol?
Alcohol consumption should be kept to a minimum when taking KEYTRUDA. You can discuss this with your healthcare professional.
Can I Exercise?
Gentle exercise, such as short walks, may help as it can help reduce constipation and the feeling of tiredness. Please discuss with your healthcare professional before starting any exercise.
Should I Use Contraception?
Yes. It is possible that your treatment could harm or cause death to your unborn baby. If you are female and able to become pregnant you should use an effective method of contraception during and for at least 4 months after the last dose of KEYTRUDA. Talk to your healthcare professional about birth control methods that you can use during this time, and tell your healthcare professional right away if you become pregnant during treatment.
Can I Breastfeed?
It is not known if KEYTRUDA passes into your breast milk. Since it is known that antibodies can be secreted in human milk, a risk to newborns/infants cannot be excluded. Please speak to your healthcare professional for further guidance if you are breastfeeding or plan to breastfeed before starting treatment.
Can I Drive And/Or Operate Machinery?
You may feel dizzy, tired or weak while taking KEYTRUDA, which can affect your ability to drive or use tools or machines. If this happens, please avoid these activities.
References:
- KEYTRUDA Patient Information Leaflet.
- Understanding MSI-H/dMMR FA.
- KEYTRUDA Summary of Product Characteristics.
- AVASTIN® (Bevacizumab) Patient Information Leaflet.
- INLYTA® (axitinib) Patient Information Leaflet.
- KISPLYX® (lenvatinib) Patient Information Leaflet.
- LENVIMA® (lenvatinib) Patient Information Leaflet.
Supporting documentation
KEYTRUDA® (pembrolizumab)
Summary of Product Characteristics | Patient Information Leaflet
GB-PDO-03167 | Date of Preparation: June 2024
Reporting of side effects: If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the package leaflet. You can also report side effects directly via the Yellow Card Scheme at https://yellowcard.mhra.gov.uk/. By reporting side effects, you can help provide more information on the safety of this medicine.


